Enduring Understanding 3.C.1: Chemical Changes and Energy
- At a macroscopic level, there are different clues that may be evidence that a chemical change has occurred.
- Chemical changes (in which changes in covalent/intramolecular bonding occur) and physical changes (involving only changes in noncovalent/intermolecular bonding) can be difficult to distinguish.
- Physical changes (water boiling to steam, solid carbon dioxide subliming to gas) involve changes in the forces between molecules, without the molecules changing their structure.
- Some changes can be 'ambiguous', such as NaCl dissolving in water. The ionic forces between the Na+ and Cl- ions are broken, but the NaCl has not undergone a chemical change, is still present, and can be regenerated by evaporating the water.
- Observations that suggest a chemical change has occurred include:
- Production of heat or light
- Formation of a gas or precipitate
- Color change.
- When chemical reactions occur, there is almost always a change in energy, which can be observed as a change in temperature of the reaction mixture.
- Exothermic reactions release energy, resulting in an increase in temperature.
- Endothermic reactions absorb energy, resulting in a decrease in temperature.
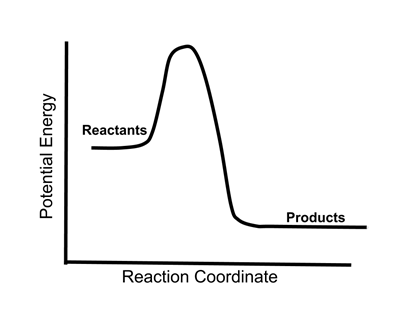
- The energy changes in a chemical reaction can be illustrated in an energy diagram.
- In the diagram above, the reactants release energy (curve lower on the vertical axis) when they become products.
- This is an exothermic reaction, which would release heat and therefore result in an increase in the temperature of the system.
- The high point in the energy curve, the transition state, represents the activation energy of the reaction. It does not affect the overall exothermic nature of the reaction.
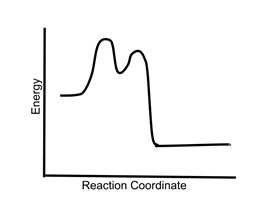
- Sample question 1: Is the following reaction exothermic, endothermic, or both?
- The reaction is exothermic. The products at the right end of the curve are lower in energy than the reactants at the left end. The presence of multiple transition states and an intermediate does not affect the energetics of the reaction.
- Sample Question 2: Given the two energy curves below, if an equivalent amount of the two reactions occurred under otherwise similar conditions, would one the mixtures be warmer than the other, or would they be the same temperature?
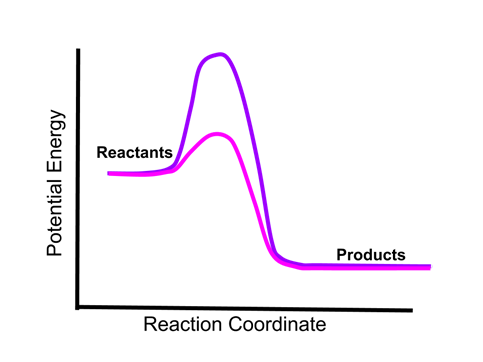
- The energy of the reactants and products indicated by the two reaction curves is the same. Therefore, the same amount of energy will be released by an equivalent amount of the two reactions, and the temperature of each reaction mixture would be the same. The height of the energy barrier (activation energy) does not affect the amount of energy absorbed or released by a reaction.
To link to this Chemical Changes and Energy page, copy the following code to your site:
