Conductors
Metals contain many electrons that are not held in an atom, these are called free electrons. This structure of metals is related to the nature of metallic bonding. Since the electrons are not being held in an atom they are free to move easily, thus allowing charge to flow.
Objects that are poor conductors of charge, such as plastics or glass, do not allow electrons to move through them. These objects are called insulators.
If two identically shaped conductors are brought into contact with one another, then electrons will flow between them until they are evenly distributed. This is because they essentially function as a single, larger conductor when they are touching. If the conductors were different shapes, then the charge will not move and the electrons will remain focused in one area.
Example.
Two identical metal spheres, each with a particular charge as shown in the diagram below, are brought together and then separated again. Determine the charge while they are touching and the charge on each after they have been separated.
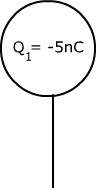
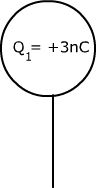
Qtouching = Q1 + Q2 Qseparated =
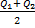
= - 5 + 3 =
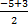
= - 2 nC = - 1 nC
|
Related Links: Science Physics |
To link to this Conductors page, copy the following code to your site:
